Design and Conduct Considerations for First‐in‐Human Trials - Shen - 2019 - Clinical and Translational Science - Wiley Online Library
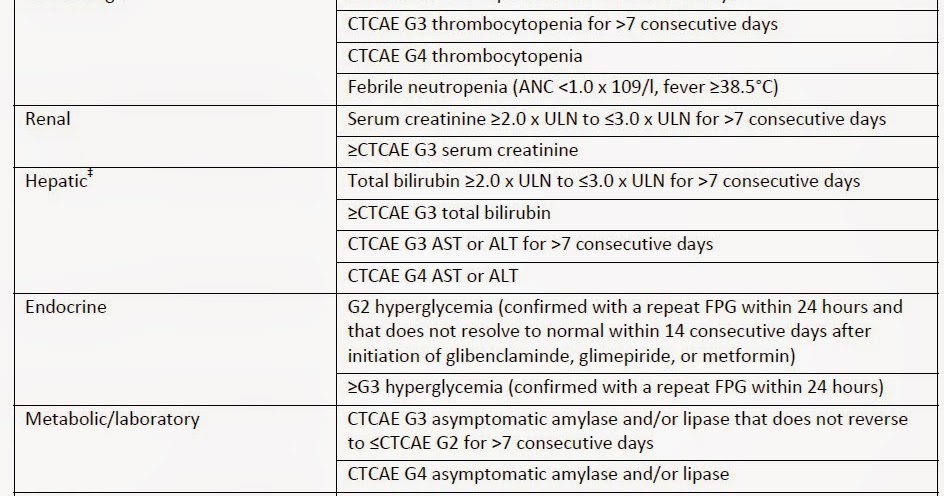
On Biostatistics and Clinical Trials: Dose Limiting Toxicity (DLT) and Common Toxicity Criteria (CTC) / Common Terminology Criteria for Adverse Events (CTCAE)

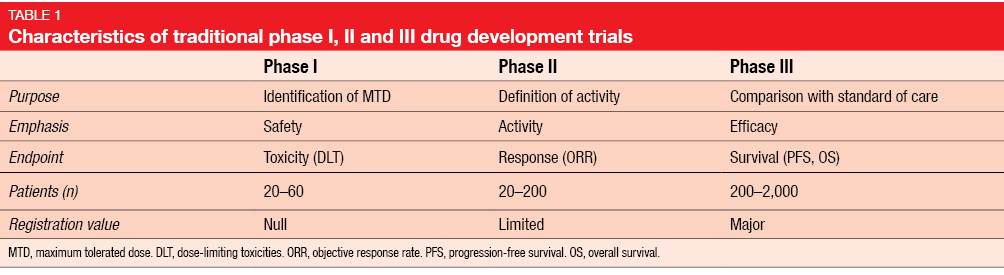
Designing Dose-Finding Phase I Clinical Trials: Top 10 Questions That Should Be Discussed With Your Statistician | JCO Precision Oncology
Application Type sNDA Application Number(s) 201023/S-20 Priority or Standard Priority Submit Date(s) November 21, 2016 Received

On Biostatistics and Clinical Trials: Dose Limiting Toxicity (DLT) and Common Toxicity Criteria (CTC) / Common Terminology Criteria for Adverse Events (CTCAE)

Illustration of the chronic dose-limiting toxicity (DLT) concept. (*)... | Download Scientific Diagram

Oncology phase I trial design and conduct: time for a change - MDICT Guidelines 2022 - ScienceDirect
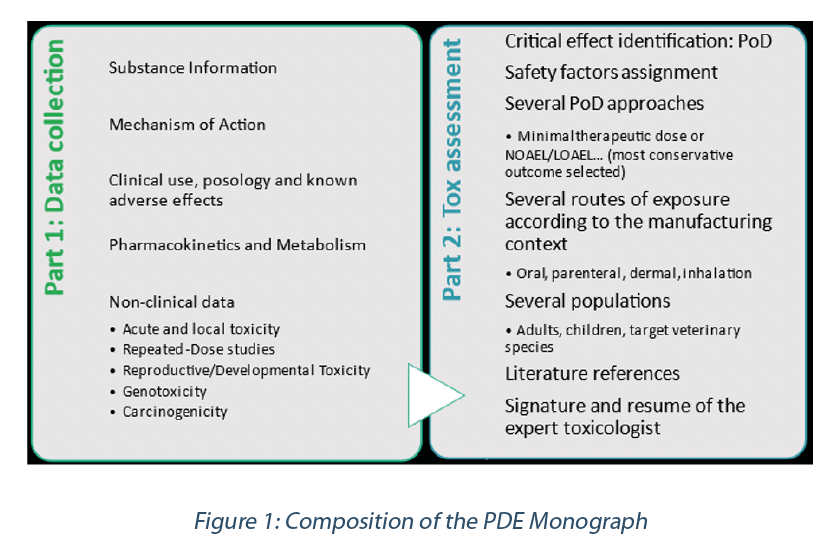
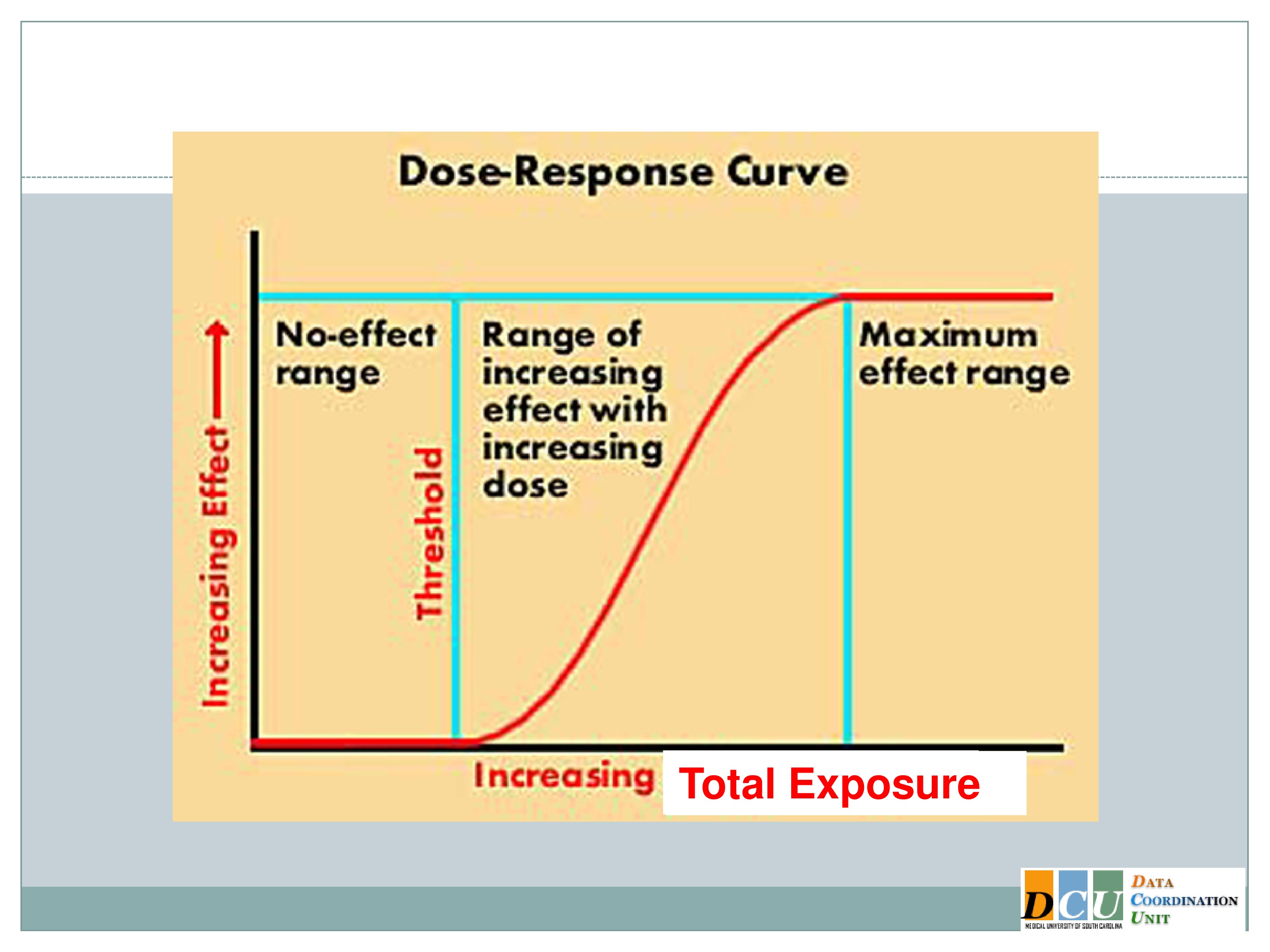
Toxicological approach to define the PDE for your cleaning validation process. - A3P - Pharmaceutical & Biotechnology Industry
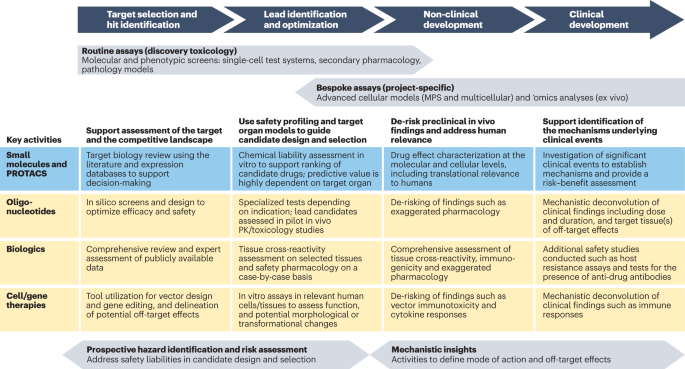
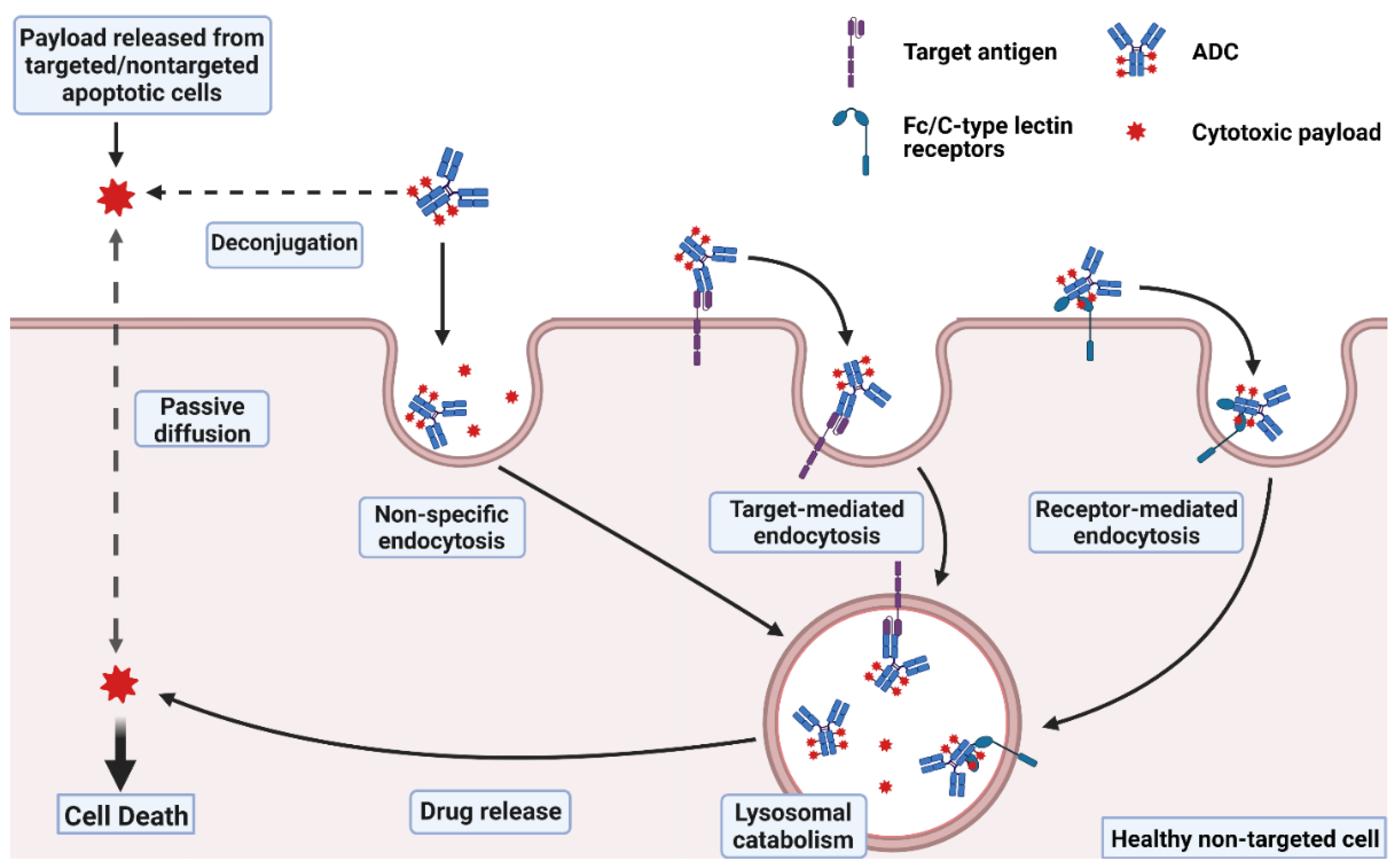
The evolving role of investigative toxicology in the pharmaceutical industry | Nature Reviews Drug Discovery

Towards new methods for the determination of dose limiting toxicities and the assessment of the recommended dose for further studies of molecularly targeted agents – Dose-Limiting Toxicity and Toxicity Assessment Recommendation Group

Prediction of Drug Approval After Phase I Clinical Trials in Oncology: RESOLVED2 | JCO Clinical Cancer Informatics

Heterogeneity in the definition of dose-limiting toxicity in phase I cancer clinical trials of molecularly targeted agents: a review of the literature. | Semantic Scholar

On Biostatistics and Clinical Trials: Maximum Tolerable Dose (MTD) and Dose-Limiting Toxicities (DLTs)
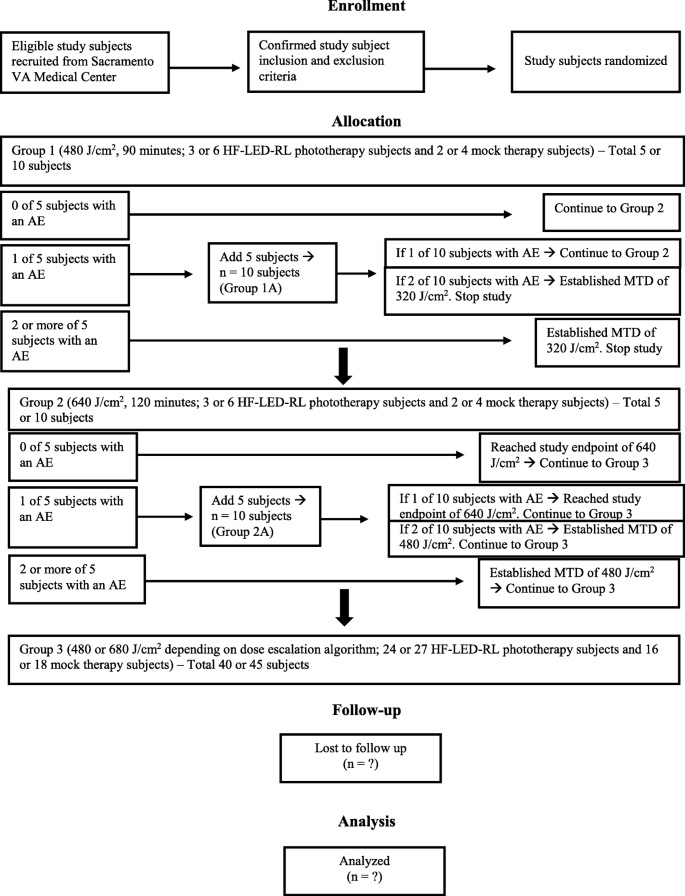
A single-blind, dose-escalation, phase I study of high-fluence light-emitting diode-red light on Caucasian non-Hispanic skin: study protocol for a randomized controlled trial | Trials | Full Text